|
Please choose one of the following....
Source: MS Encarta It is hard to believe how something which can't be pictured in the mind and which is 1 trillionth of a centimetre long was discovered before the invention of the electron microscope or the computer. The atom was not discovered over night (believe it or not). The discovery of the atom lead to many discoveries. You can now read how the atom was discovered........... CAN WE CUT THE UNCUTTABLES OF THE "UNCUTTABLE"? In ancient Greek philosophy the word atom was used to describe the smallest bit of matter that could be seen. Philosophers thought that the atom was not divisible; in fact, the Greek word for atom means “uncuttable.” Aristotle believed that the 4 elements: water, fire, air and solid, made everything in this Universe. Ptolmey believed that each substance had its own atom which had its own shape. In the 16th and 17th centuries, progress in atomic theory quickened. Chemists soon recognized that all liquids, gases, and solids can be analyzed into their ultimate components, or elements. For example, salt was found to be composed of two distinct and different elements, sodium and chlorine, which are joined together in a form known as a compound. Air was discovered to consist of a mixture of the gases nitrogen and oxygen (now we know that there are many other gases in the atmosphere, e.g. carbon dioxide and water vapour). Water was symbolized as HOH (now, its H2O), meaning that it consists of two atoms of hydrogen for every atom of oxygen. John Dalton, a British schoolmaster and chemist, was fascinated by the patchwork puzzle of the elements. Early in the 19th century he made studies of the way in which the various elements combine with one another to form compounds. Other scientists, including Isaac Newton, had already known that the smallest units of a substance are atoms. Dalton was regarded as the founder of atomic theory because he made the theory quantitative. He showed how these atoms link together in definite proportions. Subsequent investigations proved that the smallest unit of a chemical substance such as water is a molecule. Each molecule of water consists of a single atom of oxygen and two atoms of hydrogen joined by an electrical force called a “chemical bond.” There are 3 types of bonds, ionic bond (between metals and non-metals), covalent bond (between non-metals) and metallic bond (between metals).All atoms of any given element behave in the same way chemically. Thus, from a chemical viewpoint, the atom is the smallest entity to be considered. The chemical properties of the various elements are quite different; their atoms combine in many different ways to form different compounds. Some elements, such as the gases helium and argon, are inert, that is, they fail to react with other elements. Unlike oxygen, which has a diatomic molecule (two atoms combined in a single molecule), helium and other inert gases are monatomic elements, with a single atom per molecule. The study of gases attracted the attention of the Italian physicist Amedeo Avogadro, who in 1811 formulated an important law bearing his name. This law states that equal volumes of different gases contain the same number of molecules when compared under the same conditions of temperature and pressure. Given these conditions, two identical bottles, one filled with oxygen and the other with helium, will contain exactly the same number of molecules. Twice as many atoms of oxygen will be present, however, because oxygen is diatomic. If we increase temperature, volume increases, if we increase pressure, volume decreases. A "mole" is the number of atoms in 12 grams of carbon-12 (6 x 10 to the power of 24). The mass number of an element is the number of grams of one mole of this element. By the middle of the 19th century several chemists recognized that similarities in the chemical properties of various elements implied a regularity that might be illustrated by arranging the elements in a table. In 1817, Dobernier noticed that certain groups of 3 elements had very similar properties and that the r.a.m (Relative Atomic Mass) of the middle element was the average of the other 2. One example of Doberneir's triads was :lithium 7, sodium 23 and potassium 39. In 1863, Newlands arranged the elements into groups of 8 with ascending order of r.a.m. Every 8th element had similar properties and that if he arranged into groups of 7 like this: Li Be B C N O F Na Mg Al Si P S Cl K Ca Cr Ti Mn he will have 3 elements in each column like Doberneir's triads. Newlands called these groups, Newlands' octaves. The Russian chemist Dmitry Mendeleyev proposed a chart of elements called the periodic table, in which the elements are arranged in rows and columns so that elements with similar chemical properties are grouped together. According to this arrangement, each element was assigned a number (atomic number) ranging from 1 for hydrogen to 92 for uranium. Because not all the elements were known at the time of Mendeleyev, blank spaces were left in the periodic table, each of which corresponded to a missing element. Further research, aided by the arrangement of the known elements in the chart, led to the discovery of missing elements. Elements of higher atomic number have correspondingly heavier atomic weights. Elements in a row (or Period) had the same number of electron shells, elements in a column (or Group) had the same number of electron in their outer shells. Curiosity about the size of the atom and its weight tantalized hundreds of scientists for a long period during which lack of adequate instruments and proper techniques prevented them from obtaining satisfactory answers. Subsequently, a variety of ingenious experiments was devised to determine the size and weight of the various atoms. The lightest of all atoms, hydrogen, has a diameter of 1 × 10-8 cm (0.00000001 cm) and weighs 1.7 × 10-24 (the fraction of a gram represented by 17 preceded by 23 zeros and a decimal point). An atom is so small that a single drop of water contains more than a million million billion atoms.
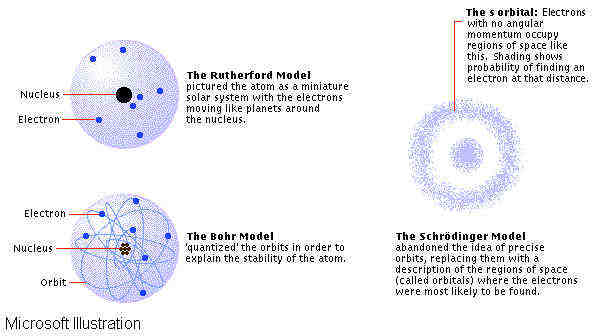
The electron was discovered by Sir Joseph Thomson. Electrons were believed to be particles. Thomson won the Noble Prize for this discovery. Ten years later, his son won the Noble Prize for proving his father wrong, electrons could also be waves. Thomson thought that an atom was a ball with one big positive cloud with small electrons in it. This was called the "pudding" model. In vacuum tubes a heated cathode emits a stream of electrons that can be used to amplify or rectify an electric current. If such a stream is focused into a well-defined beam, it is called a cathode-ray beam. Cathode rays directed against suitable targets produce X rays; directed against the fluorescent screen of a television tube, they produce visible images. Also, the negatively charged beta particles emitted by some radioactive substances are electrons. Electrons have a rest mass of 9.109 x 10-28 grams, and an electrical charge of negative 1.602 x 10-19 coulombs. The charge of the electron is the basic unit of electricity. Electrons are classified as fermions because they have half-integral spin; spin is a property of subatomic particles that indicates the particle's angular momentum. In 1909, Hans Geiger, Ernst Marden and Ernst Rutherford were working in Manchester University studying a type of radiation called alpha particles. They were watching alpha particles scattered by a metal foil. Most of the alpha particles went through the metal foil. Rutherford concluded that most of the space of an atom was empty and that 99% of its mass was concentrated in its centre which he called the nucleus. He knew that this nucleus was positive. He found out alpha particles were actually helium atoms(two protons and a neutron). He also proposed that satellites called electrons travel in orbits around the nucleus. The nucleus has a positive charge of electricity; the electrons each have a negative charge. The charges carried by the electrons add up to the same amount of electricity as in the nucleus, and thus the normal electrical state of the atom is neutral. Otto Hahn managed to split the atom by shooting a neutron at a uranium atom. The atom split in 2, releasing a lot of energy. The mass of the 2 atoms was a little bit less than the mass of the uranium atom. This mass was turned into energy. Without this discovery, the atomic bomb wouldn't have been invented.
The neutron was first identified in 1932 by the British physicist Sir James Chadwick, who correctly interpreted the results of experiments conducted at that time by the French physicists Irène and Frédéric Joliot-Curie and other scientists. The Joliot-Curies had produced what Chadwick recognized as neutrons by the interaction of alpha particles with beryllium nuclei. When this newly discovered radiation was passed through paraffin wax, collisions between the neutrons and the hydrogen atoms in the wax produced readily detectable protons. The neutron is a particle found in all nuclei of mass number greater than 1; that is, of all nuclei except ordinary hydrogen. Free neutrons—those outside of atomic nuclei—are produced in nuclear reactions. They can be ejected from atomic nuclei at various speeds or energies and are readily slowed down to very low energy by a series of collisions with light nuclei, such as those of hydrogen, deuterium, or carbon. When expelled from the nucleus, the neutron is unstable and decays to form a proton, an electron, and a neutrino. Like the proton and the electron, the neutron possesses angular momentum, or spin. Neutrons act as small, individual magnets; this property enables beams of polarized neutrons to be created. The neutron has a negative magnetic moment of -1.913141 nuclear magnetrons. Its half-life (the time it takes for half of a group of neutrons to disappear) was fixed approximately at 10.61 minutes. One of
the great successes of theoretical physics was the explanation of the characteristic
line spectra of various elements. Atoms excited by a supply of energy
from an external source emit light of well-defined frequencies. If hydrogen
gas, for example, is held at low pressure in a glass tube and an electrical
current is passed through it, visible light of a reddish color is given
off. Careful examination of this light with a prism spectroscope shows
a line spectrum, a series of regularly spaced lines of light, each of which
has a definite wavelength and associated energy. The Bohr theory permits
the physicist to calculate these wavelengths in a straightforward fashion.
It is assumed that in the hydrogen atom the outer electron can move in
stable orbits. While the electron remains in an orbit at a fixed distance
from the nucleus, the atom does not radiate energy. When the atom is excited,
the electron jumps to a higher-energy orbit farther from the nucleus, and
as it falls back to its normal orbit, it emits a discrete amount of energy
corresponding to a certain wavelength of light. Each line of light observed
represents an electronic transition between a higher and lower energy orbit.
In many heavier elements, if an atom is sufficiently excited so that inner
electrons close to the nucleus are affected, then penetrating radiation,
or X rays, will be emitted. These electronic transitions involve large
amounts of energy. This theory explains reflection (when atoms absorb energy
and reflect energy as light) and transparency (when electrons absorb some
energy but lets most of it travel through it).
Experimental data has been the impetus behind the creation and dismissal of physical models of the atom. Rutherford's model, in which electrons move around a tightly packed, positively charged nucleus, successfully explained the results of scattering experiments, but was unable to explain discrete atomic emission—that is, why atoms emit only certain wavelengths of light. Bohr began with Rutherford’s model, but then postulated further that electrons can only move in certain quantized orbits; this model was able to explain certain qualities of discrete emission for hydrogen, but failed completely for other elements. Schrödinger’s model, in which electrons are described not by the paths they take but by the regions where they are most likely to be found, can explain certain qualities of emission spectra for all elements; however, further refinements of the model, made throughout the 20th century, have been needed to explain all observable spectral phenomenon. Accelerator studies eventually established that each kind of particle also has an antiparticle of the same mass but opposite in charge or other electromagnetic property. Physicists have long sought a theory that would put this bewildering array of particles in order. Particles are now grouped according to the force that usually controls their interactions. Hadrons (strong nuclear force) include hyperons, mesons, and the neutron and proton. Leptons (electromagnetic and weak forces) include the tau, muon, electron, and neutrinos. Bosons (particle like objects associated with interactions) include the photon and the hypothetical carriers of the weak force and of gravitation. The weak nuclear force is evident in such radioactive or particle-decay reactions as alpha decay (the release of a helium nucleus from an unstable atomic nucleus). See Antimatter. In 1963 the U.S. physicists Murray Gell-Mann and George Zweig proposed that hadrons are actually combinations of more fundamental particles called quarks, the interactions of which are carried by particle like gluons. This theory underlies current investigations and has served to predict the existence of further particles.Accelerator studies eventually established that each kind of particle also has an antiparticle of the same mass but opposite in charge or other electromagnetic property. Physicists have long sought a theory that would put this bewildering array of particles in order. Particles are now grouped according to the force that usually controls their interactions. Hadrons (strong nuclear force) include hyperons, mesons, and the neutron and proton. Leptons (electromagnetic and weak forces) include the tau, muon, electron, and neutrinos. Bosons (particle like objects associated with interactions) include the photon and the hypothetical carriers of the weak force and of gravitation. The weak nuclear force is evident in such radioactive or particle-decay reactions as alpha decay (the release of a helium nucleus from an unstable atomic nucleus). See Antimatter. In 1963 the U.S. physicists Murray Gell-Mann and George Zweig proposed that hadrons are actually combinations of more fundamental particles called quarks, the interactions of which are carried by particle like gluons. This theory underlies current investigations and has served to predict the existence of further particles. CAN WE CUT THE UNCUTTABLES OF THE ATOM? A quark is any of six hypothetical particles that are believed to form the basic constituents of the elementary particles called hadrons, such as the proton, neutron, and pion. The quark concept was proposed in 1963 by two different researchers, American physicists Murray Gell-Mann and George Zweig. (The term quark was taken from Irish writer James Joyce's Finnegan's Wake.) Quarks were first classified as three kinds: up, down, and strange. The proton, for example, is believed to be constituted of two up quarks and one down quark. Later theorists postulated the existence of a fourth quark; in 1974 the existence of this quark, named charm, was experimentally confirmed. Thereafter a fifth and sixth quark—called bottom and top, respectively—were hypothesized for theoretical reasons of symmetry. Experimental evidence for the existence of the bottom quark was obtained in 1977. The top quark eluded researchers until March 1995, when two teams of physicists at Fermi National Accelerator Laboratory (Fermilab) announced they had detected and measured the top quark. Each kind of quark has its antiparticle, and each kind of quark or antiquark comes in three types of “colors.” Quarks can be either red, blue, or green, while antiquarks can be either antired, antiblue, or antigreen. These quark and antiquark colors have nothing to do with the colors seen by the human eye. Rather, these colors represent a quantum property. When combining to form hadrons, quarks and antiquarks can only exist in certain color groupings. The hypothetical carrier of the force between quarks is called the gluon. One reason why it will be difficult to discover quarks is that the force between 2 quarks increases as the distance between them increases. |