Contents:-
Introduction
Early Theories
Bronsted-Lowry Theory
Measuring Acid or
Base Strength
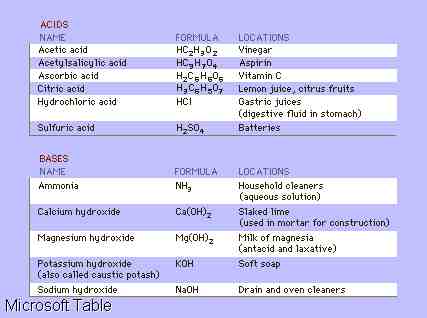
Acids and Bases, two classes
of chemical compounds that display generally opposite characteristics.
Acids taste sour, turn litmus (a pink dye derived from lichens) red, and
often react with some metals to produce hydrogen gas. Bases taste bitter,
turn litmus blue, and feel slippery. When aqueous (water) solutions of
an acid and a base are combined, a neutralization reaction occurs. This
reaction is characteristically very rapid and generally produces water
and a salt. For example, sulfuric acid and sodium hydroxide, NaOH, yield
water and sodium sulfate:
H2SO4
+ 2NaOH2H2O + Na2SO4
Early Theories
Modern understanding of
acids and bases began with the discovery in 1834 by the English physicist
Michael Faraday that acids, bases, and salts are electrolytes. That is,
when they are dissolved in water, they produce a solution that contains
charged particles, or ions, and can conduct an electric current (Ionization).
In 1884 the Swedish chemist Svante Arrhenius (and later Wilhelm Ostwald,
a German chemist) proposed that an acid be defined as a hydrogen-containing
compound that, when dissolved in water, produces a concentration of hydrogen
ions, or protons, greater than that of pure water. Similarly, Arrhenius
proposed that a base be defined as a substance that, when dissolved in
water, produces an excess of hydroxyl ions, OH-. The neutralization reaction
then becomes:
H+
+ OH-H2O
A number of criticisms
of the Arrhenius-Ostwald theory have been made. First, acids are restricted
to hydrogen-containing species and bases to hydroxyl-containing species.
Second, the theory applies to aqueous solutions exclusively, whereas many
acid-base reactions are known to take place in the absence of water.
Brønsted-Lowry
Theory
A more satisfactory theory
was proposed in 1923 by the Danish chemist Johannes Brønsted and
independently by Thomas Lowry, a British chemist. Their theory states that
an acid is a proton (hydrogen ion, H+) donor and a base a proton acceptor.
Although the acid must still contain hydrogen, the Brønsted-Lowry
theory does not require an aqueous medium. For example, liquid ammonia,
which acts as a base in aqueous solution, can act as an acid in the absence
of water by transferring a proton to a base and forming the amide anion
(negative ion) NH2-:
NH3
+ baseNH2- + base + H+
The Brønsted-Lowry
definition of acids and bases also explains why a strong acid displaces
a weak acid from its compounds (and likewise for strong and weak bases).
Here acid-base reactions are viewed as a competition for protons. In terms
of a general chemical equation, the reaction of Acid (1) with Base (2)
Acid (1) + Base (2)Acid
(2) + Base (1)
results in the transfer
of a proton from Acid (1) to Base (2). In losing the proton, Acid (1) becomes
its conjugate base, Base (1). In gaining a proton, Base (2) becomes its
conjugate acid, Acid (2). The equilibrium represented by the equation above
may be displaced either to the left or to the right, and the actual reaction
will take place in the direction that produces the weaker acid-base pair.
For example, HCl is a strong acid in water because it readily transfers
a proton to water to form a hydronium ion:
HCl + H2OH3O+
+ Cl-
The equilibrium lies mostly
to the right because the conjugate base of HCl, Cl-, is a weak base, and
H3O+, the conjugate acid of H2O,
is a weak acid.
In contrast, hydrogen
fluoride, HF, is a weak acid in water because it does not readily transfer
a proton to water:
HF + H2OH3O+
+
F-
This equilibrium lies mostly
to the left because H2O is a weaker base than F-, and because HF is a weaker
acid (in water) than H3O+. The Brønsted-Lowry theory also explains
why water can be amphoteric, that is, why it can serve as either an acid
or a base. Water serves as a base in the presence of an acid that is stronger
than water (such as HCl), in other words, an acid that has a greater tendency
to dissociate than does water:
HCl + H2OH3O+
+ Cl-
Water can also serve as
an acid in the presence of a base that is stronger than water (such as
ammonia):
NH3+
H2ONH4+ + OH-
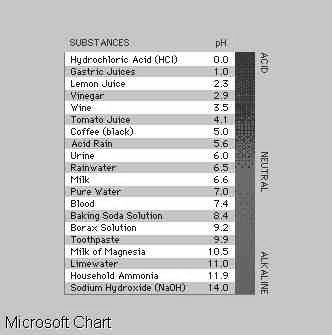
Measuring Acid or Base
Strength
The strength of an acid
can be measured by the extent to which an acid transfers a proton to water
to produce the hydronium ion, H3O+. Conversely, the
strength of a base is indicated by the extent to which the base removes
a proton from water. A convenient acid-base scale is calculated from the
amount of H3O+ that is formed in water solutions of
acids or of OH- formed in water solutions of bases. The former is known
as the pH scale and the latter as the pOH scale (pH).
The
value for pH is equal to the
negative logarithm of the hydronium ion concentration-and for pOH, of the
hydroxyl ion concentration-in an aqueous solution:
pH = -log [H3O+]
pOH = -log [OH-]
Pure water has a pH of
7.0. When an acid is added, the hydronium ion concentration [H3O+]
becomes larger than that in pure water, and the pH becomes less than 7.0,
depending on the strength of the acid. The pOH of pure water is also 7.0,
and in the presence of a base, the pOH drops to values lower than 7.0.
The American chemist Gilbert
N. Lewis has offered another theory of acids and bases that has the further
advantage of not requiring the acid to contain hydrogen. This theory states
that acids are electron-pair acceptors and bases are electron-pair donors.
This theory also has the advantages that it works when solvents other than
water are involved and it does not require the formation of a salt or of
acid-base conjugate pairs. Thus, ammonia is viewed as a base because it
can donate an electron pair to the acid boron trifluoride, for example
H3N:
+ BF3H3N-BF3
to form an acid-base
association pair.

